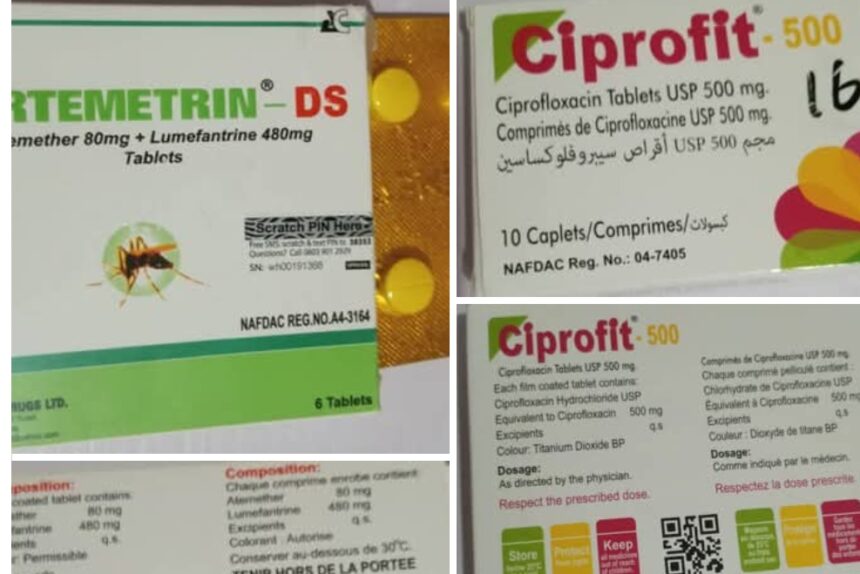
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert on the circulation of substandard and falsified medicines in Nigeria.
In a statement on Wednesday, the agency identified two counterfeit products—ARTEMETRIN DS (Artemether/Lumefantrine) tablets (80mg/480mg) and CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg)—both falsely labelled as manufactured in Enugu State.
Preliminary checks using Thin-Layer Chromatography (TLC) raised concerns about the quality of the drugs, prompting further analysis at a World Health Organization (WHO)-prequalified laboratory. The advanced tests confirmed the medicines were grossly substandard.
According to NAFDAC, ARTEMETRIN DS contained only 59.2% Artemether and 71.2% Lumefantrine, well below the acceptable 90–110% limits, while CIPROFIT 500 contained just 5.7% of Ciprofloxacin instead of the standard range.
Both products were reportedly purchased from a “licensed vendor and wholesaler” but do not exist on the official NAFDAC registered products database. The registration numbers printed on their packaging were also confirmed to be false.
NAFDAC has urged the public to immediately stop using or selling these medicines and to return any stock to the nearest NAFDAC office. Consumers who may have taken the drugs and experienced adverse reactions are advised to seek urgent medical attention.
Healthcare professionals and the general public have been encouraged to report suspected cases of falsified or substandard medicines to NAFDAC through its toll-free line 0800-162-3322 or via email at sf.alert@nafdac.gov.ng.